Resolution on drugs and medical supplies: Applying while waiting for instructions
(Baonghean.vn) - Resolution 30/NQ-CP initially removed some of the difficulties and obstacles in solving the shortage of drugs, medical equipment, and medical supplies in medical facilities... However, there are still many shortcomings.
On March 4, 2023, the Government issued Resolution No. 30/NQ-CP on continuing to implement solutions to ensure medicine and medical equipment to remove difficulties and obstacles for hospitals in paying health insurance costs, purchasing medicine, supplies, etc.
Resolving difficulties for hospitals
Resolution No. 30/NQ-CP allows continued payment of medical examination and treatment costs under health insurance for technical services performed using machines provided by contractors (installed or borrowed electrical equipment).GovernmentAllow contracts signed before November 5, 2022 to be implemented according to the contract term; contracts signed from November 5, 2022 will be implemented until there is a new legal document, including contracts signed in the form of direct procurement. In case the contract term as prescribed above expires, payments will continue until the purchased materials and chemicals are used up.
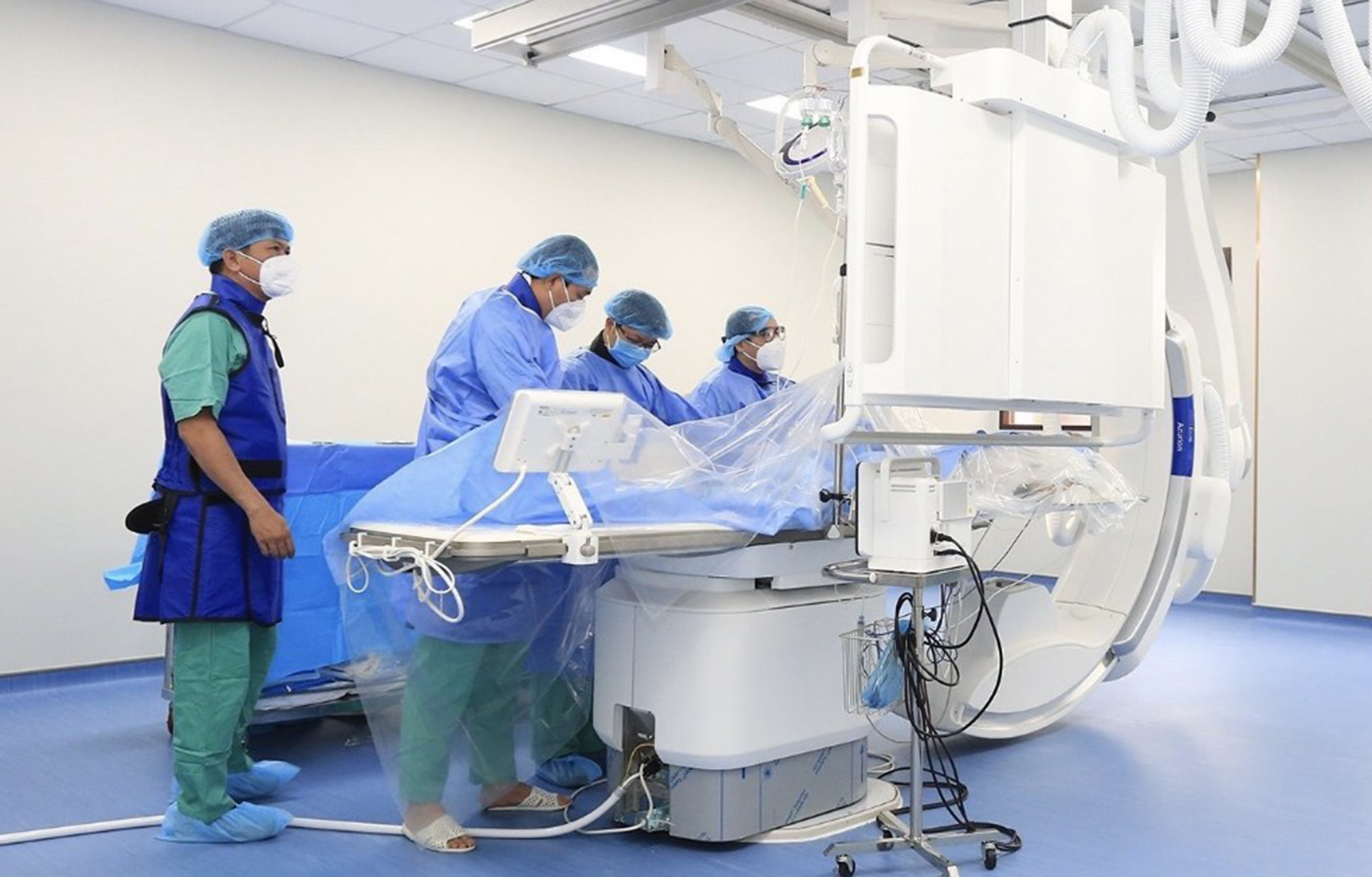 |
Resolution No. 30 allows continued payment of medical examination and treatment costs under health insurance for technical services performed by machines provided by contractors. Photo: Internet |
Before Resolution 30/NQ-CP was issued, this was a very difficult point due to the requirement of not paying insurance for services performed on contracted equipment signed after November 5, 2022, because the hospital currently does not have new equipment and does not have financial resources to invest in new purchases.
Resolution No. 30/NQ-CP allows medical facilities to pilot the application of guidelines on building bidding packages in 2023. Specifically, when building bidding packages, in cases where the same type of medical equipment has many manufacturers, the investor will consider and decide to assign the unit's scientific council to build features and technical configurations according to the unit's professional requirements, thereby obtaining quotes and determining bidding packages.
The basis for determination includes: Market price is referenced from the quotations of suppliers. After the public announcement of the price, at least 10 days, the investor shall base on the number of quotations received as the basis for establishing the bid package price... In case there are only 1 or 2 distributors providing quotations, the received quotations shall be used as the basis for establishing the bid package price. The investor shall be allowed to obtain quotations directly from the distributor in case there is only 1 distributor or to ensure compatibility in technology and copyright that cannot be purchased from other distributors.
This is also a regulation to remove obstacles, because the old regulation required "3 quotes" in purchasing and repairing, while many medical devices only have 1-2 supplier representatives in Vietnam, so hospitals cannot buy equipment, and broken equipment cannot be repaired.
Resolution No. 30/NQ-CP also allows medical facilities to use medical equipment that has been donated, given, donated, contributed, aided, or sponsored by domestic or foreign individuals and organizations, but has not yet completed the procedures for establishing public ownership for medical examination and treatment. Technical services performed using this medical equipment are paid for by the health insurance fund. Medical examination and treatment facilities are responsible for the quality of medical services provided from this medical equipment and are allowed to use the facility's budget for maintenance, repair, and repair when in use.
It is known that in the past, due to problems in receiving donated equipment, many hospitals could not receive donations of surgical microscopes and surgical and treatment equipment worth tens of billions of dong.
Resolution No. 30/NQ-CP clearly assigns the Ministry of Health the responsibility to develop and promulgate guidelines on developing prices for medical equipment bidding packages (must be completed by the second quarter of 2023).
The Ministry of Health shall amend and supplement the list of drugs for centralized bidding at the national and local levels to ensure that it is consistent with the bidding organization capacity of each level and the supply capacity of enterprises; research and develop a mechanism to ensure rare drugs and drugs with limited supply; develop and promulgate sample bidding documents for bidding packages of medicinal materials and traditional medicines for online bidding... (to be completed in the third quarter of 2023).
Review and specifically evaluate the receipt of used medical machinery and equipment donated, given, contributed, sponsored, or aided by organizations and individuals for medical facilities to use for medical examination and treatment; on that basis, coordinate with the Ministry of Finance and relevant ministries and agencies to propose specific solutions to report to competent authorities for consideration and decision (To be completed in the second quarter of 2023).Many problems and still have to wait
Immediately after Resolution 30/NQ-CP,Ministry of Healthorganized a conference to guide the implementation to health departments, hospitals, medical facilities and medical equipment manufacturing and trading enterprises. Next, Nghe An Department of Health also organized a conference to deploy to medical facilities in the province. The purpose of the conferences is to thoroughly grasp the content of the resolution, to avoid the situation where each medical facility understands and applies the new regulations in a different way.
 |
To be able to shop "comfortably", medical facilities still have to wait for specific instructions from the Ministry of Health and related ministries and branches. Photo: Internet |
In these conferences, health departments and health units all agreed that Resolution 30/NQ-CP initially partially resolved the difficulties and obstacles in solving the shortage of drugs, medical equipment, and medical supplies of health facilities in the work of purchasing, bidding, payment, and settlement of medical examination and treatment costs under health insurance... However, there are still many shortcomings.
Firstly, the guidelines in Resolution 30/NQ-CP are only temporary and short-term. The pilot policies in the resolution can be "withdrawn" at any time, and do not create a solid enough legal foundation for hospitals to confidently implement. And in reality, when the resolution does not meet legal requirements, hospitals are afraid to do it, and then will be held responsible. This is a health economic problem, not a medical professional problem. The long-term "root" solution is to amend the law, from which decrees and circulars will be issued. In the case of amending the law, the most important thing is the Law on Bidding, at least there must be a separate chapter regulating drugs and medical equipment.
Second, with the new regulation, there will be no need to have 3 quotations in the bidding process for chemicals and equipment, but without a reference price, monopolistic enterprises may push up the prices of chemicals and equipment. In addition, it is difficult for medical facilities to know whether the quotations of enterprises are close to the imported prices from abroad or not. Therefore, the Ministry of Finance needs to closely monitor the prices of enterprises to protect those who work in procurement and bidding.
Third, the most lacking aspect in the current bidding for equipment procurement is the legal aspect. Therefore, in the implementation process, it is necessary to deploy Resolution 30/NQ-CP to other sectors so as not to cause difficulties for units of the health sector in the process of inspection and examination.
Fourth, the Resolution has not resolved the problems for district-level medical facilities. Because the bidding documents require the winning bidder to provide equipment for use of materials and chemicals. However, because district-level hospitals typically have few patients, low demand for testing and service use, the winning bidder is concerned that the machines will not operate at the right capacity, resulting in poor business performance, causing difficulties in bidding. Thus, bidding at the district level is still very difficult.
With these difficulties and shortcomings, it is clear that in order to be able to "comfortably" shop, medical facilities still have to wait for specific instructions from the Ministry of Health and related ministries and branches. In addition, the Resolution has assigned the Ministry of Health to amend and supplement the list of drugs for centralized bidding at the national and local levels (to be completed in the third quarter of 2023), which is still a situation of "water far away cannot put out a nearby fire" for the current drug shortage.
In the conference guiding the implementation of Resolution 30/NQ-CP, the Ministry of Health also said that the difficulties and problems cannot be completely resolved immediately but must be resolved step by step. The Ministry of Health will continue to listen to the opinions of medical units and facilities about difficulties and problems in the practice of purchasing and bidding for medical equipment, supplies, drugs, etc. The leaders of the Nghe An Department of Health directed the departments and offices of the department to continue reviewing the difficulties and problems of the units to propose to the Ministry of Health and the Government to come up with solutions.
Pharmacist Tran Minh Tue - Deputy DirectorNghe An Department of Healthclearly stated: While waiting for the Ministry of Health to guide the implementation of Resolution 30/NQ-CP, medical facilities must be truly responsible, proactive in planning, organizing bidding for the purchase of drugs, chemicals, and medical supplies not on the list of centralized procurement to serve medical examination and treatment in 2023; not to let there be a shortage of drugs, chemicals, and medical supplies and people have to buy drugs from outside. Units must carefully study, grasp, and thoroughly understand the content of the Resolution; seriously implement it in accordance with regulations. The Department of Health has established 10 support groups, units need to continuously connect and report problems for timely direction./.
