Ministry of Health announced 21 types of fake drugs
Of the 21 products seized, four were identified as counterfeit drugs licensed for circulation by the Ministry of Health.
This warning was sent by the Ministry of Health to local Health Departments today, in the context of Thanh Hoa Provincial Police dismantling a large-scale fake medicine production and trading ring nationwide and arresting 14 people.
Of the 21 products seized, four were identified as counterfeit drugs licensed by the Ministry of Health, including: Tetracycline, Clorocid, Pharcoter and Neo-Codion. That is, the information on the labels of these products or the names of the drugs were completely identical to the real drugs licensed by the Ministry of Health.
"Local health departments and health sectors urgently notify businesses and drug users not to trade, sell, or use these fake products," said the Drug Administration of Vietnam.
Specifically, the labels of 4 types of counterfeit drugs have the following information:
Clorocid TW3 Tablets(Chloramphenicol 250mg), Registration number: VD-25305-16; Manufacturer: TW3 Pharmaceutical Joint Stock Company, packaged in plastic bottles of 400 tablets.
Tetracycline TW3 Tablets(Tetracycline hydrochloride 250mg), Registration number: VD-28109-17; Manufacturer: Central Pharmaceutical Joint Stock Company 3, packaged in plastic bottles of 400 tablets.
Pharcoter tablets(Codein base 10mg; Terpin hydrate 100mg), Registration number: VD-14429-11; Manufacturer: TW1 Pharmaceutical Joint Stock Company (Pharbaco), packaged in plastic bottles of 400 tablets.
Counterfeit Neo-Codion drug products. This drug does not have any information on the label. However, the Drug Administration of Vietnam said that the drug Neo-Codion licensed for circulation by the Ministry of Health has the following official information: circulation license number 300111082223 (old registration number: VN-18966-15); active ingredient Codein base (in the form of Codein camphosulfonate 25mg) 14.93mg; Sulfogaiacol 100mg; Grindelia soft extract 20mg; dosage form is sugar-coated tablets; packaged in boxes of 2 blisters x 10 tablets. Manufacturer: Sophartex Company (France), address: 21, rue du Pressoir, Vernouillet, 28500.
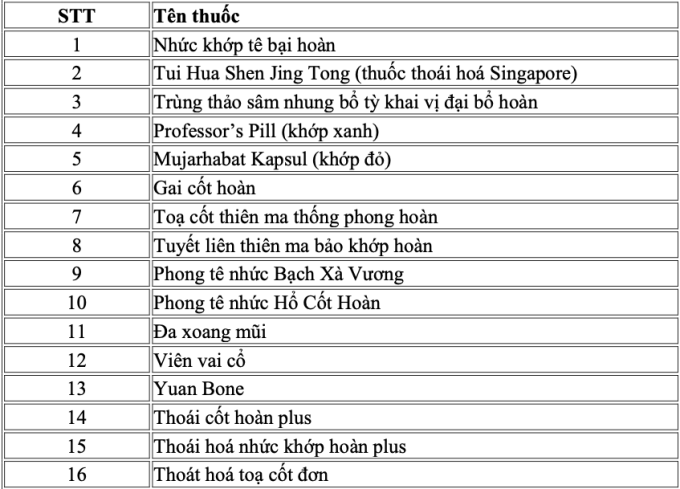
The remaining 16 products do not match any of the drugs on the list that have been granted a registration license by the Ministry of Health. The list includes: Rheumatoid arthritis; Tui Hua Shen Jing Tong (Singaporean degenerative medicine); Cordyceps ginseng velvet antler spleen stimulant great bo hoan; Professor's Pil (green joints); Mujarhabat Kapsul (red joints); Gai cot hoan...
In addition to publishing and warning about the list of counterfeit drugs, the Ministry of Health requires hospitals to review the drug procurement and supply process, ensuring that they only use drugs with circulation licenses, provided from legal sources and with full invoices and documents.
When detecting suspicious or unlicensed drugs, it is necessary to immediately seal them, stop using them and report to the authorities for handling according to regulations.
People are advised to only buy medicine at legal businesses, avoid using medicine of unknown origin and report any suspicious signs of counterfeit medicine to authorities.
Localities need to set up hotlines to receive information about counterfeit and smuggled drugs to trace their origins and strictly handle violations.
On April 17, the Ministry of Health said that "counterfeit drugs cannot penetrate public hospitals" because they do not have documents and certificates to participate in bidding. 21 types of counterfeit drugs were produced in Hanoi, Ho Chi Minh City and An Giang. Of these, there were 4 types of counterfeit modern medicine (44 boxes of Tetracycline, 40 boxes of Clorocid, 49 boxes of Pharcoter, 52 boxes of Neo-Codion); 39,323 boxes of 17 types of counterfeit products suspected to be oriental medicines, products with labels stating the intended use as medicine.
