Ministry of Health speaks out about banned drug that killed 1 person and left 3 in critical condition
On August 19, the Ministry of Health confirmed that the results of a search of the licensing database at the Drug Administration of Vietnam showed that so far in Vietnam there are no drugs containing the active ingredient Phenylbutazone with a valid circulation registration certificate.
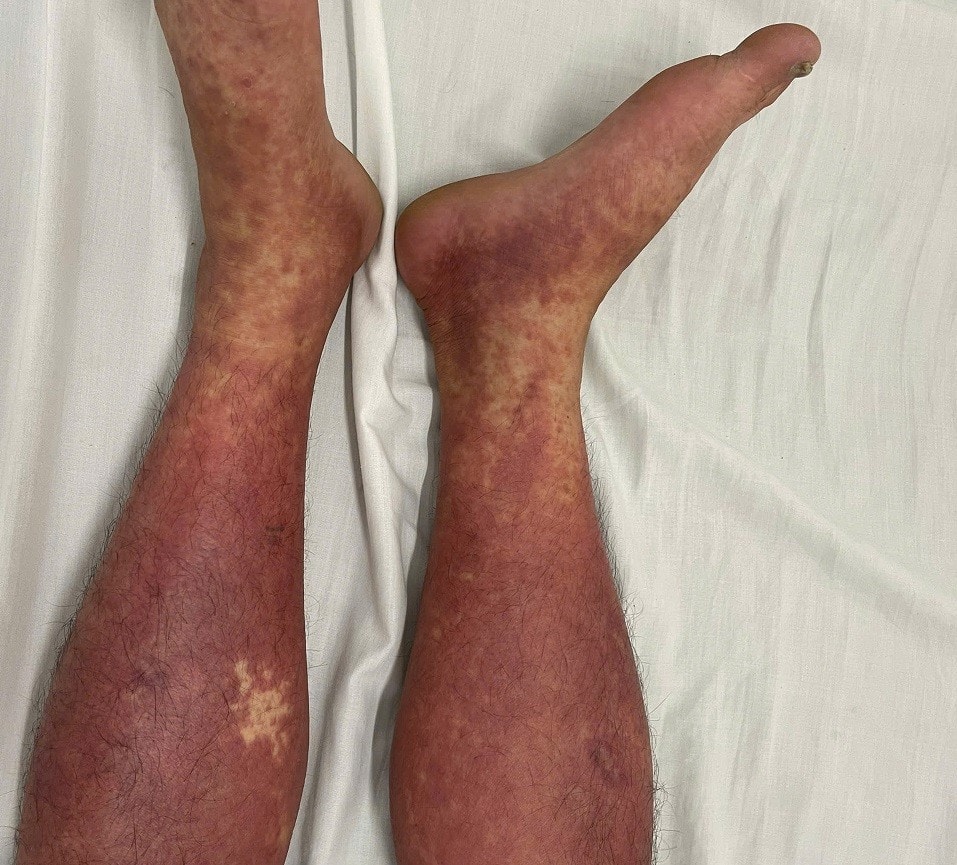
Bach Mai Hospital recently issued an urgent warning regarding the series of severe drug allergy cases since the beginning of 2025 due to Phenylbutazone, a non-steroidal anti-inflammatory drug, which has long been used to treat acute gout and ankylosing spondylitis, but has been banned by the Ministry of Health since 2001.
Of these, 1 person died and 3 people were in critical condition due to self-medication. The 4 patients included 3 men and 1 woman, the youngest of whom was only 39 years old. The fatal case was a 70-year-old woman in Phu Tho.
Although it has been banned for a long time, according to VietNamNet's research, on e-commerce platforms such as Shopee and Lazada, drugs containing Phenylbutazone are still widely sold for the treatment of arthritis, especially acute gout. The selling price ranges from 170,000 - 220,000 VND/box depending on the store.
There are accounts that advertise this product as hand-carried from China, not on the list of prohibited goods and safe for users.

Regarding products containing Phenylbutazone of unknown origin, Mr. Ta Manh Hung, Deputy Director of the Department of Drug Administration, Ministry of Health, on August 19, affirmed that according to the results of searching the licensing database at the Department, up to now in Vietnam there are no drugs containing the active ingredient Phenylbutazone with a valid circulation registration certificate.
"The Drug Administration does not grant permission to import Phenylbutazone raw materials or finished drugs containing this substance," said Mr. Hung.
Doctor Chu Chi Hieu, Head of the Allergy Department, Center for Allergy & Clinical Immunology, Bach Mai Hospital, said Phenylbutazone is a banned drug because of the risk of causing severe allergies, multiple organ failure, and even the patient's life.
To ensure safety for users, the Drug Administration of Vietnam requires the Department of Health of 34 provinces/cities to notify all drug trading and using establishments and people to absolutely not buy, sell or use drugs containing Phenylbutazone or any drugs of unknown origin or drugs not licensed for circulation.
"When discovering products containing Phenylbutazone circulating on the market, please immediately notify the Department of Health and relevant agencies for timely inspection and handling according to regulations," said the leader of the Drug Administration of Vietnam.
The Departments of Health are also requested to continue to coordinate with competent authorities (Steering Committee 389, police, market management...) to strengthen inspection, investigation, verification and strict handling of cases of buying and selling drugs of unknown origin, especially cases of advertising and selling drugs online in violation of regulations.
For pharmaceutical businesses and medical examination and treatment facilities with websites, the Drug Administration of Vietnam requires reviewing all information posted on the websites and removing advertising information that is not in accordance with regulations about products containing Phenylbutazone to avoid misunderstanding that this is a drug licensed for use in Vietnam.


.jpg)

