Ministry of Health requests withdrawal of salbutamol ingredient in 9 drugs
Director of the Drug Administration of Vietnam (Ministry of Health) Truong Quoc Cuong said that this unit has just issued Official Dispatch No. 19197/QLD-DK on removing salbutamol ingredient in 9 types of drugs from the List of active pharmaceutical ingredients allowed to be imported without requiring an import license for domestic drugs that have been granted registration numbers.
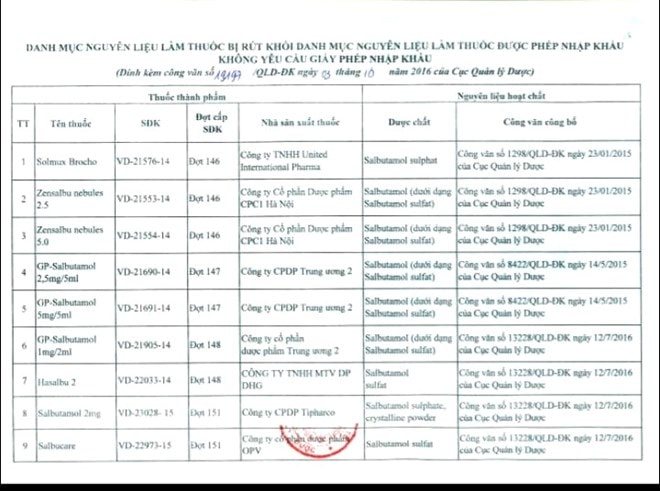 |
| List of 9 drugs that use the active ingredient salbutamol. |
The Drug Administration has notified domestic drug registration and production companies about withdrawing salbutamol from 9 drugs using this active ingredient.
These are the drugs: Solmux Brocho (registration number: VD-21576); Zensalbu nebules 2.5 and 5.0 (VD- 21553 and 2554); GP- Salbutamol content 2.5mg/5ml, 5mg/5ml and 1mg/2ml (VD – 21690; 21691; 21905); Haslbu (VD- 22033); Salbutamol 2mg (VD- 23028); Salbucare (VD-22973).
The above drugs are produced by United Internationa Pharma Limited Liability Company; Hanoi CPC1 Pharmaceutical Joint Stock Company; Central Pharmaceutical Joint Stock Company 2; DHG Pharmaceutical One Member Co., Ltd....
According to the Drug Administration of Vietnam, salbutamol (a bronchodilator drug commonly used in cases of respiratory diseases accompanied by bronchospasm) has been an active ingredient used as a medicine to treat humans for many years.
Finished drugs containing Salbutamol are used in the medical industry mainly in the respiratory department with indications for testing respiratory function, treating asthma attacks, preventing bronchospasm caused by exertion, treating severe asthma attacks, chronic bronchitis, and bronchiectasis./.
According to Vietnamplus


