Discover the secret of the hardest material on the planet, harder than diamond
Russian scientists have just designed a new model for a material based on fullerene crystals with extremely high mechanical hardness, even higher than diamond.
This discovery offers the potential to deepen our understanding of fullerene materials, advance research in this field, and may help synthesize superhard materials for the processing industry.
A team of researchers from the Russian National University of Science and Technology (MISiS), the Institute of Technology of New Carbon and Superhard Materials in the Russian capital, and several other Russian universities and research institutes have discovered how fullerite crystals turn into a superhard material, several times harder than diamond. The team has also come up with a model for the structure of this special material.
The study was published in the journal Carbon.
Fullerite is a molecular crystal structure, with a "lattice" of fullerene molecules.
Fullerenes are a new type of molecule in which the atoms form a hollow sphere. The carbon spheres in fullerites can be arranged in a variety of patterns, although the hardness of the material depends largely on how the fullerenes are connected.
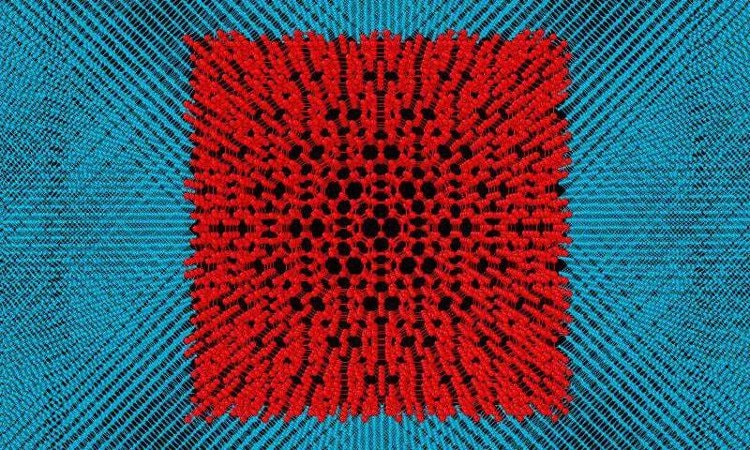 |
| Fullerite model in diamond. (Photo source: Sputnik). |
Researchers around the world have created models of polymerization (in the process of creating a polymer, a low molecular weight substance is attached to the active center of a polymer molecule) to convert fullerenes into fullerites. Previously, it was not clear why the created materials are superhard, as was found in some tests. Russian scientists have succeeded in explaining this process.
Russian researchers surrounded a fullerite crystal structure with single-crystal diamond and then studied the compound that was created from it.
The hypothesis put forward by the Russian researchers is that the compression of fullerite at high temperatures converts some of the fullerenes into polycrystalline diamonds, while the other part remains in the compressed form (SH phase).
According to calculations by Russian scientists, the fullerite inside the diamond must be compressed, as compression enhances its elasticity and mechanical properties, while the diamond shell keeps the fullerite in place and preserves its properties.
"Unlike diamond, fullerite is not a single-crystalline material. Under normal conditions, this material is completely soft. However, during the 3D polymerization process under high pressure, its compressive strength and hardness increase significantly, and the material becomes superhard. Fullerite can then increase the compressive strength of the diamond surface to 310 GPa. We believe that these properties are due to the compressed state of fullerite," said Dr. Pavel Sorokin, PhD in Physics and Mathematics.
Dr. Sorokin heads the Theoretical Materials Science project on Nanostructures at the MISiS Laboratory of Inorganic Nanomaterials. He also heads the laboratory at the Institute of Technology of New Carbon and Superhard Materials in Moscow.
Researchers believe they may soon be able to solve the mystery of superhard materials. The results obtained through computer modeling could help create stronger fullerite-based variants, and produce them in large enough quantities to meet demand and replace diamond as the main carbon material used in today's processing industry.
According to VOV
| RELATED NEWS |
|---|




.jpeg)



