Nghe An warns about fake Agifamcin 300 capsules
(Baonghean.vn) - Nghe An Department of Health has issued an official dispatch directing medical examination and treatment units and pharmaceutical businesses to stop distributing and recall fake Agifamcin 300 capsules.
Implementing the Official Dispatch of the Drug Administration Department - Ministry of Health on the recall of Agifamcin 300, batch number 00916, HD 22/3/2019. To ensure safety for users, Nghe An Department of Health issued Official Dispatch No. 1726/SYT-QLD dated July 13, 2018 directing all medical examination and treatment units, pharmaceutical businesses, private pharmacies in the province to stop distributing, using and recall at their facilities, return to the unit that provided the above drug and report to the Inspection Department or the Drug Administration Department - Department of Health.
 |
| Agifamcin 300 medicine. Photo: Document |
According to the Drug Administration of Vietnam - Ministry of Health, through the supervision of the production and trading activities of Agifamcin 300, the inspection team of the Department discovered that there were some boxes of fake Agifamcin in circulation on the market, with the label bearing the name of the manufacturer Agimexpharm Pharmaceutical Joint Stock Company and the following information: Agifamcin 300 capsules, registration number VD-14223-11, batch number 00916, expiry date March 22, 2019.
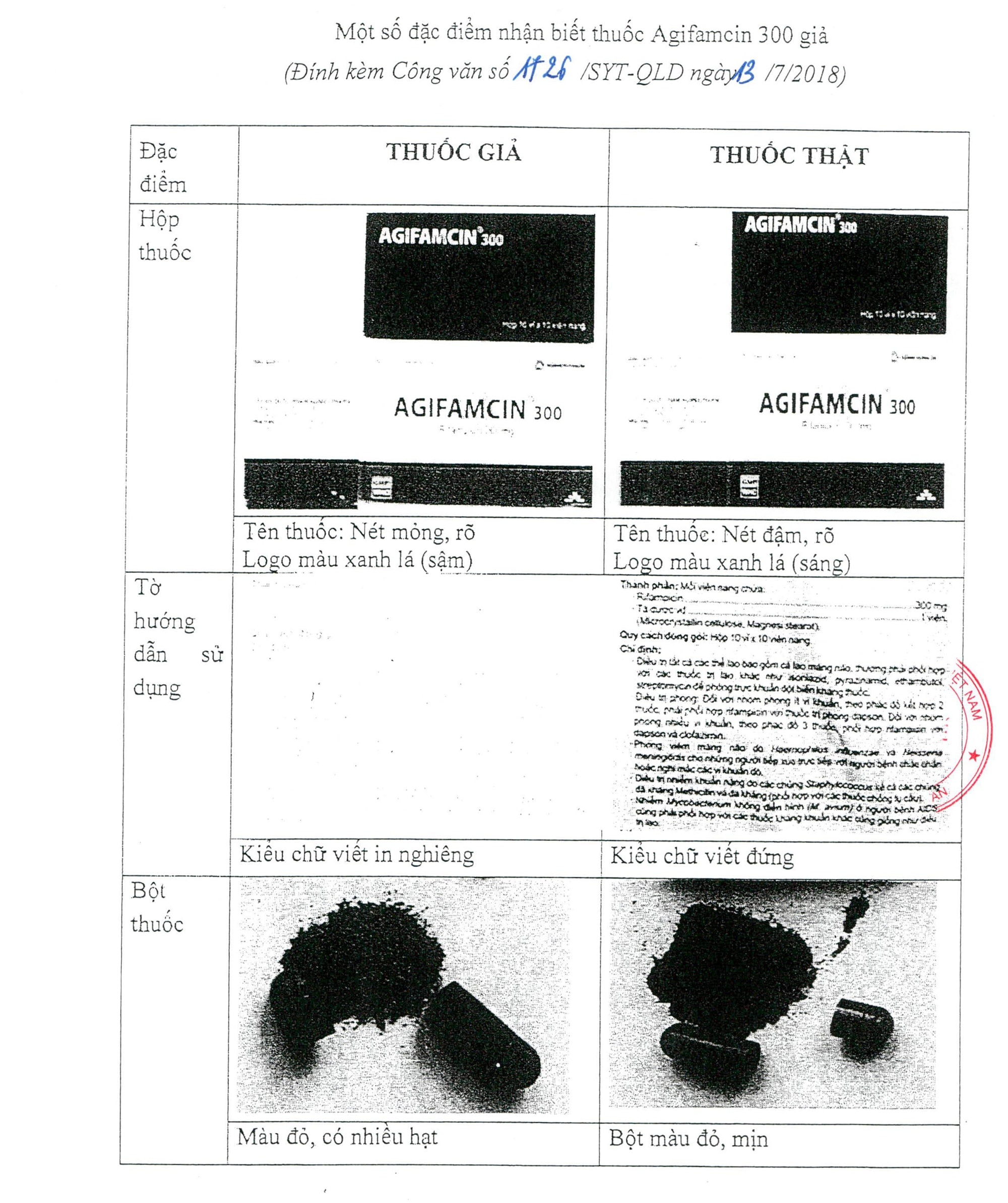 |
| Content of the Department of Health's dispatch distinguishing between real and fake drugs. Photo: Provided by the Department of Health's Communications Department |
This medicine is distributed simultaneously with the real medicine Agifamcin 300, registration number VD-14223-11, batch number 00916, expiry date 3/22/2019, manufactured by Agimexpharm Pharmaceutical Joint Stock Company.
It is known that Agifamcin capsules are antiparasitic, antibacterial, antiviral, and antifungal drugs often prescribed to treat tuberculosis and leprosy.

