Vietnam's Covid-19 vaccine creates very good immunity, preparing for phase 2
The first three volunteers received a 25mcg booster dose. The vaccine initially produced a very good immune response.
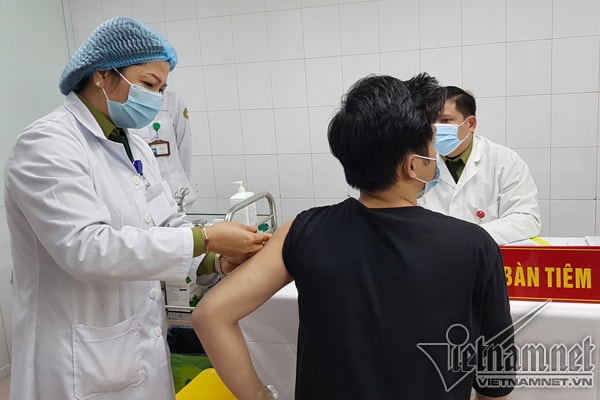
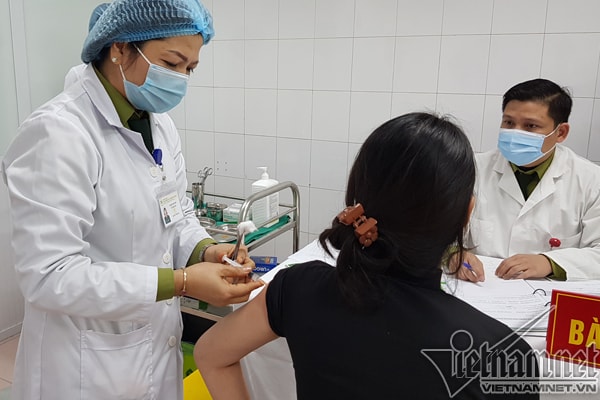
On the morning of January 14, the Military Medical Academy began administering the second dose.Nanocovax vaccine25mcg dose for the first 3 volunteers of group 1 (including 20 people) 28 days after the first injection.
The first 3 volunteers include 1 male and 2 females, aged from 21 to 42. The remaining 17 people in group 1 are expected to receive the second injection on January 17.
After the second injection, all three volunteers were in stable health and stayed at the Military Medical Academy for 24 hours of monitoring before returning home. After the first injection, two-thirds of the volunteers had a mild fever and pain at the injection site, but all went away after 24 hours, while the remaining cases had no reactions.
 |
| Research staff prepare a 25mcg dose of Nanocovax vaccine before injection. |
Also today, 10 more volunteers will be injected with the first dose of Nanocovax vaccine 75mcg, 1 day earlier than the original plan with the permission of the Ministry of Health.
Associate Professor, Dr. Ho Anh Son - Deputy Director of the Military Medical Research Institute,Military Medical AcademyThe research team said that they had injected the vaccine into more than 40 volunteers in all three dose groups of 25mcg, 50mcg and 75mcg. After monitoring, the volunteers' health was stable and their mental state was completely normal.
After vaccination, blood samples were taken from volunteers on days 7, 14 and 21 for the research team to assess immunogenicity and virus neutralization ability.
“Our initial results show that the Nanocovax vaccine is very safe and creates a very good immune response. After the second injection, the amount of antibodies will continue to increase 4-5 times, even 20 times,” Associate Professor Son informed.
 |
 |
 |
| The first 3 volunteers in Vietnam received the second injection of the Nanocovax vaccine produced by Nanogen. |
The research team will continue to monitor the volunteers' health for another 6 months and 1 year to assess whether the amount of antibodies is sustainable and how long they last.
“The study has gone more than halfway through phase 1, so we are processing data and sending ethics documents to the Ministry of Health to determine the appropriate dose for phase 2. Most likely, phase 2 will start right after Tet and maintain 2 dose levels,” Associate Professor Son said.
According to Associate Professor Son, compared with selection standardsvolunteersUnlike many major pharmaceutical companies in the world, Vietnam requires stricter criteria. There was a volunteer from Dong Thap who waited 5 days to get vaccinated, but after 3 tests, one index failed, so he had to go home.
In phase 1, because the research focuses on evaluating the safety of the vaccine and finding the optimal dose, volunteers must have a BMI of 18-28 and have never had a hypersensitivity or allergic reaction.
However, in phase 2, the criteria will be much broader, requiring 560 volunteers to participate.



