New crystalline material could double solar cell efficiency
A new material recently announced by researchers at Purdue University and the National Renewable Energy Laboratory (NREL) in the US shows that it has the ability to generate twice as much electricity as silicon, the main material currently used to make solar panels.
Limitations of silicon solar cells
| Silicon solar cells have a maximum efficiency of only 33%. |
Because silicon's band gap is so large, very few valence electrons receive enough energy from photons to become free electrons. In addition, in silicon, the lifetime of these free electrons is very short (only about 1 picosecond - or 10-12 seconds) and the distance they travel is only 10 nanometers.
After traveling the above distance, the free electrons lose all the energy provided by the photons (from the Sun). As a result, the efficiency of silicon solar cells is very low because most of the received light energy is converted into heat energy.
Perovskite crystal material solution
To overcome this problem, Libai Huang, assistant professor of chemistry at Purdue, and his colleagues developed a new technique based on microscopes and fast lasers to track the distance and speed of free, energetic electrons in the crystal lattice.
Using this technique, they were able to record the properties of many different materials. Ultimately, what the scientists found was a material tentatively named "hybrid perovskite". This material is made from lead (Pb), iodine (I) and methyl-ammonium (CH3NH3).
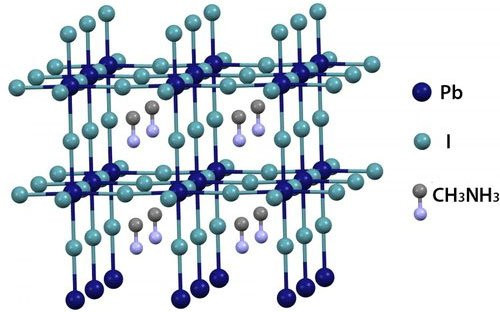 |
| Crystal structure of hybrid perovskite materials. |
The special feature of the above material is that it has a crystal structure similar to perovskite (CaTiO3), a type of compound with a unique structure consisting of "cages" surrounding the free atoms located in the center. With hybrid perovskite, the Pb and I atoms form "cages" surrounding the CH3NH3 cluster located in the center. It is this perovskite structure that allows free electrons to travel longer distances and survive longer before losing all their energy.
Ms. Huang described more specifically: "The distance that free electrons need to travel is at least equal to the thickness of the solar panel (to generate electricity), about 200 nanometers. This is the number that the new perovskite material can achieve. In addition, these electrons can survive up to 100 picoseconds, 100 times longer than silicon."
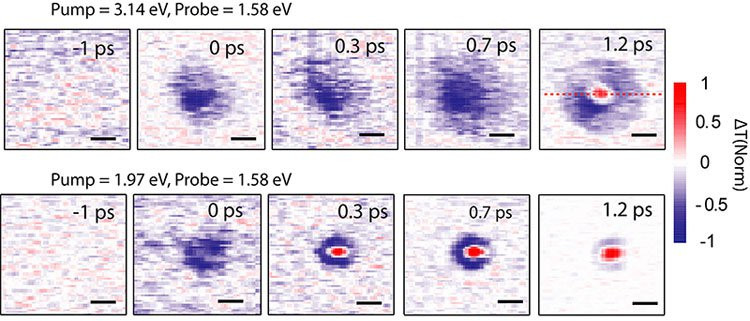 |
| Fast laser images show the lifetime of free electrons in the crystal lattice. |
Kai Zhu, a co-author of the report from NREL, was excited by the results: "This research shows that free electrons carrying energy in a standard perovskite crystalline thin film can travel a distance longer than or equal to the thickness of the panel, which is necessary to make an efficient solar cell. This information also indicates that the potential for developing solar cells using perovskite structures is very good."
However, one drawback of this material is that it uses Pb, an environmentally toxic element. Purdue researchers are now trying to develop a material with a similar perovskite structure but without the Pb. And the final detail is to perfect the product. Mr. Zhu summed up: "The next step is to find or develop the right material or structure with the right energy levels to extract these free electrons to create current in the secondary circuits. This will not be simple."
According to Khoahoc.tv
| RELATED NEWS |
|---|


