Hemostatic device appears in just 20 seconds
When used, these absorbent pellets will expand 10 times larger and seal the wound, stopping bleeding in less than 20 seconds.
The US military's 20-second syringe-shaped device has been cleared by the US Food and Drug Administration (FDA) for civilian use, rather than just confined to hospitals on the battlefield. With normal wounds taking 3 or 4 minutes to completely stop bleeding, the device has a promising future when widely applied to the US people.
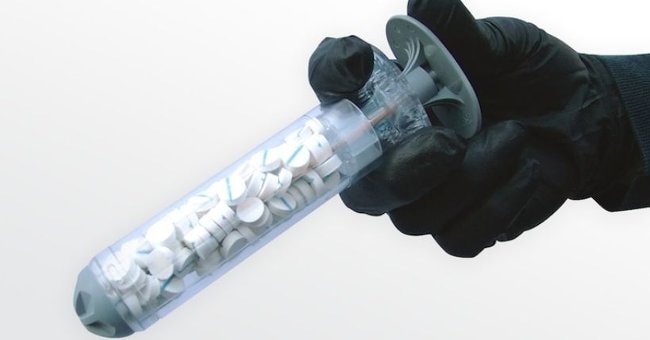
Called the XStat 30, the device is capable of sealing dangerous open wounds caused by gunshots or stab wounds. It is especially useful for wounds in places where bandages cannot hold tightly, such as under the armpits or near the groin.This hemostatic device is manufactured by medical device company RevMedx and was officially used in the military in April 2015.
 |
| XStat is not suitable for wounds that appear in some dangerous areas. |
XStat consists of a 30mm diameter plastic cylinder and 92 special absorbent pellets, each measuring 9.8mm and capable of absorbing 3ml of blood. When used, these absorbent pellets will expand 10 times larger and tightly seal the wound, stopping bleeding in less than 20 seconds. The absorbent pellets are made from synthetic cellulose, coated on the outside with chitosan - which helps increase blood clotting and prevent infection. They can replace cotton bandages within 4 hours, giving patients enough time to get to the hospital for surgery.
XStat has now been approved for public health use in the United States. This unique device will be a timely life-saving solution for bleeding people who have had serious accidents or have been attacked by lethal weapons. According to FDA statistics, nearly 50% of soldiers participating in combat have fallen into a state of severe blood loss and many have died before reaching the nearest medical facility.
"The purpose of XStat is to improve first aid for bleeding patients who cannot be taken to medical facilities," said Andrew Barofsky, CEO of RevMedx. In addition, William Maisel, a senior FDA official, added: "We are eager to see this technology applied to life to help people control the situation of patients dying from excessive blood loss." Although highly appreciated, the FDA itself also recommends that XStat is not suitable for wounds appearing in some dangerous areas such as: chest, pelvis, abdomen or above the collarbone.
According to info.net
| RELATED NEWS |
|---|


