High-priced drugs: Lack of supervision allows businesses to freely set prices?
The National Assembly's Committee on Social Affairs assessed that drugs paid for by the Health Insurance Fund (accounting for over 50% of the total number of drugs) are currently regularly monitored by the social insurance agency, while drugs purchased by people themselves or purchased by hospitals for medical examination and treatment not paid for by the Health Insurance Fund are branded drugs and exclusive drugs that are often very expensive because there is no mechanism to monitor price transparency.
According to Ms. Truong Thi Mai - Chairwoman of the National Assembly's Committee on Social Affairs, the revised Law on Pharmacy needs to have a more effective mechanism to control the prices of these drugs as well as ensure a balance between the interests of businesses (when the drugs are under copyright protection) and the rights of patients to avoid causing "disasters due to high medical costs."
As we have seen, drug prices vary in many places and many types of specialty drugs have "sky-high" prices. Are the right people and diseases treated or are there still loopholes for patients to be "pickpocketed"?
The draft revised Law on Pharmacy, which is being submitted to the National Assembly for approval in the near future, has once again "dug up" the story of drug prices, in which there are many changes expected to better control the prices of pharmaceuticals. Will this become a reality or is it still just control at the policy and strategy level?
Amended Pharmacy Law: Transparent drug price management
In fact, although the Pharmaceutical Law has been in effect for 10 years, the domestic pharmaceutical market still depends heavily on foreign countries. Currently, pharmaceutical enterprises import 90% of pharmaceutical ingredients and 50% of the total drugs used in the country are foreign drugs.
 |
| Currently, domestic pharmaceutical enterprises import 90% of raw materials for making drugs. |
Notably, according to statistics from the Ministry of Health, in 2014, on average each Vietnamese person spent 31 USD on medicine and 50% of this amount was used to buy imported medicine (mostly at high prices). And in reality, in the current pharmaceutical market, there is still a situation where medicine prices are high in some places and low in others.
According to the Government's Report on the Draft Law on Amended Pharmacy, after 10 years of implementation, many provisions in the Law on Pharmacy are no longer suitable to reality and the process of international integration, and need to be studied and amended. The Law has not yet stipulated policies to create motivation for the development of the pharmaceutical industry such as ensuring output for domestically produced drugs, developing medicinal materials, producing vaccines, biological products, etc.
That inadequacy has led to the situation where Vietnam still depends on imports to meet the people's drug needs. In addition, a series of inadequacies in state management of drug prices were also pointed out by National Assembly deputies, especially the lack of transparency in prices.
Minister of Health Nguyen Thi Kim Tien affirmed that, in addition to the achieved results, in the process of implementing the 2005 Pharmacy Law, there are still some difficulties and problems arising from reality, because some provisions of the Law are no longer suitable to reality and the process of international integration, and need to be studied and amended.
The head of the health sector cited examples such as: Regarding drug price management, the Pharmaceutical Law only assigns the Ministry of Health as the focal point without assigning tasks among ministries and sectors in drug price management, so the implementation process encounters many difficulties and does not ensure transparency, while drug price management requires multi-sectoral coordination.
According to the representative of the Ministry of Health, for the above reasons, the development and promulgation of the revised Law on Pharmacy is extremely necessary. The development of the revised Law on Pharmacy Project aims to promote socialization, mobilize all resources for this activity, and ensure adequate supply of quality drugs at reasonable prices.
Drug prices: Still just hope!
Regarding the revised Law on Pharmacy, according to Ms. Truong Thi Mai - Chairwoman of the National Assembly's Committee on Social Affairs: The Committee finds that the draft Law has stipulated the principles of drug price management according to the market mechanism, respecting the right of enterprises to self-determine prices, ensuring publicity and transparency. The State only intervenes to stabilize prices for essential drugs when drug prices fluctuate abnormally, affecting socio-economic stability, and at the same time assigns responsibility for state management of drug prices.
In general, the provisions on drug price management of the draft Law are consistent with the Law on Prices and the Law on Bidding. Accordingly, the drug price management mechanism is clearer, especially the provisions on price negotiation for proprietary drugs, new drugs, drugs under copyright, centralized drug bidding and preferential policies in selecting contractors as domestic drug manufacturers.
In the revised Law on Pharmacy, drug bidding will have some main revised contents such as giving priority to generic drugs and domestic drugs; centralized bidding at the national level and some drugs centralized at the provincial level, even decentralized prescriptions for centralized bidding at the local level.
Regarding the price management agency, the Ministry of Health will be responsible to the Government for price management. However, many opinions say that there needs to be a clear division of responsibilities among relevant ministries and branches to properly implement the Law on Bidding and the Law on Prices.
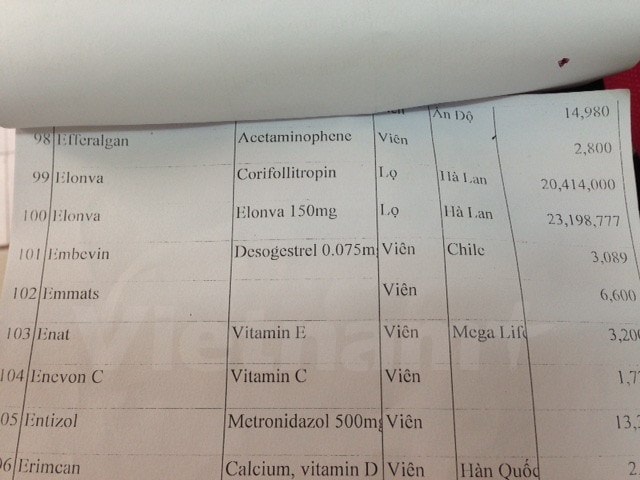 |
| Medicines cost up to tens of millions of dong in the price list at a central hospital. |
Mr. Do Van Dong - Deputy Director of the Department of Drug Administration (Ministry of Health) also pointed out that in the revised Law on Pharmacy, instead of having to declare and post prices according to the law, with the price level being equal to that of countries with the same level of medical care and similar economy - which is not feasible - the health sector must follow two forms: following the market and managing prices publicly and transparently with drugs provided by the budget.
Thus, the revised Pharmaceutical Law is expected to fundamentally overcome the large price difference between localities, and there will be no situation of declaring prices too high. This form will be public, transparent, and collected to ensure adequate supply of quality and acceptable prices, and also in accordance with current regulations of countries on price management and drug bidding processes.
Drug price negotiations: Implementation will be difficult
According to the Ministry of Health, to limit the situation of increasing prices of exclusive generic drugs, the draft Law stipulates that the procurement of generic drugs must comply with the provisions of the Bidding Law. Accordingly, for generic drugs and drugs with few suppliers in the market, the form of national price negotiation will be applied.
This is a new method added in the 2013 Law on Bidding, this method has been applied by many countries in the world to manage the prices of branded drugs, drugs with few suppliers on the market. Accordingly, price negotiation is applied to drugs with only one or two manufacturers; branded drugs, rare drugs, drugs under copyright and other special cases.
Price negotiations will be conducted at the national level by the National Price Negotiation Council. The application of the price negotiation mechanism will ensure uniformity in prices of branded drugs, and through the negotiation process, will ensure reasonable prices based on comparisons and assessments with other countries in the world and related costs.
In addition, the draft Law on Pharmacy has added solutions to increase competition to reduce the price of branded drugs: allowing early registration applications before the patent of an invented drug expires in Clause 5, Article 7; incentives and support for drug manufacturing enterprises to invest in the production of new branded drugs whose patents have expired.
Analyzing this issue, Mr. Nguyen Van Tien - Vice Chairman of the National Assembly's Committee on Social Affairs emphasized that branded drugs are copyrighted drugs of the manufacturer, so they must comply with the manufacturer's price and the state has a price negotiation policy. For example, if a drug enters Vietnam through only 1 or 2 distributors, the state will negotiate the price with that supplier. When they sell in the Vietnamese market, they need to sell at a reasonable price so that people will support and have many buyers, while a high price will have few buyers.
The Vice Chairman of the National Assembly's Committee on Social Affairs cited that some cancer and hepatitis drugs are very expensive and there are only 1-2 suppliers. If Vietnam does not allow them to import, there will be no alternative drugs and the people will still be disadvantaged. Therefore, the role and responsibility of the health sector is to organize negotiations with suppliers about their sales to the Vietnamese market and "check" how much they sell to other countries, from there negotiate prices to reduce the disadvantage of the people.
Mr. Tien emphasized: “Currently, the new law has not been implemented in any cases. The implementation will be difficult because this is the first time we have implemented it. It is not difficult, but we have to learn from experience to reduce mistakes. If we do it too quickly and carelessly, it will not work. Next year, we will implement price negotiation and centralized bidding, with fewer price negotiations, about 10-15 types of specialty drugs, and price negotiations will certainly be effective.”
According to Vietnamplus
| RELATED NEWS |
|---|








