Emergency suspension of cardiovascular drug with fatal risk
The Drug Administration of Vietnam - Ministry of Health has announced the suspension of circulation and recall of all finished drugs containing the active ingredient buflomedil because the drug causes serious adverse reactions on the cardiovascular and nervous systems, sometimes leading to death.
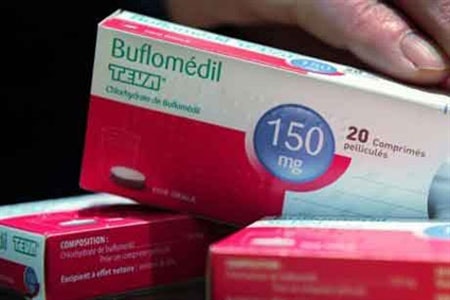
All finished products containing the active ingredient buflomedil are being recalled.
The Drug Administration of Vietnam requires drug manufacturing, importing, distributing and trading units to urgently recall all drug batches containing the active ingredient buflomedil nationwide and requires these units not to import, produce and use raw materials containing the active ingredient buflomedil.
For treatment facilities, the department requires to immediately stop prescribing and using drugs containing the active ingredient buflomedil.
Previously, the Drug Administration Department announced the suspension of nationwide circulation of dospirin film-coated tablets (aspirin 81mg - antipyretic, analgesic effect), production batch: 1201002; expiry date: 6.2.2015; registration number: VD-12548-10 produced by SPM Joint Stock Company because it did not meet quality standards and quantitative indicators and required the company to coordinate with the distributor to recall the entire batch of this drug.
According to LDO-M






