Turns Out What We Drink Every Day Is the Weirdest Liquid in the Universe
We have learned a lot about water and its properties. But not everyone realizes that these properties are so unique that even if we traveled all over the universe, we would have difficulty finding a second substance.
For us humans, water is a substance that is both familiar and strange. Familiar because water covers 71% of the Earth's surface, is present in most living organisms, and accounts for 70% of the human body mass...
But water is also very strange because of its special properties that, until now, scientists have not been able to fully explain.

For us humans, water is a substance that is both familiar and strange.
First things firstmust mention the density of water. When the temperature decreases, most liquids will contract. But water only obeys this rule as long as the temperature is above 4 degrees Celsius. If the temperature is lower, the water will expand more as the temperature decreases. That also means, water has the greatest density at 4 degrees Celsius.
Special featuresNext is ice. When we put ice in water, we see that the ice always floats on the surface. This phenomenon is so common in life that you often ignore it.
But note that a substance in solid state is usually denser than it is in liquid state. So, shouldn't ice sink in water?

Rocks float on water.
Not only that, water has a boiling point and surface tension much greater than many other liquids. Water also dissolves very well. Therefore, water has great biological significance and is known as"solvent of the universe".
In an attempt to explain the peculiar properties of water, researchers from the University of Bristol (UK) and the University of Tokyo (Japan) used a supercomputer to reconstruct a model of the water molecule. They discovered that at room temperature, water molecules have a tetrahedral structure.
It is this pyramidal arrangement of atoms that gives water abilities that most other liquids do not have.
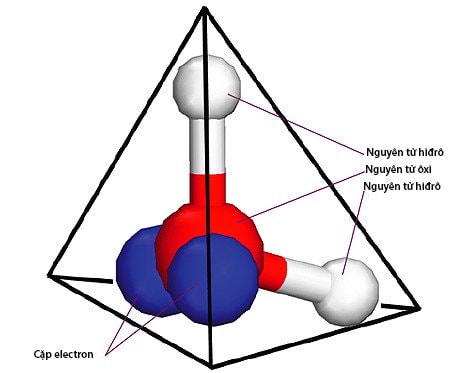
Tetrahedral structure of water molecule.
In further experiments, experts simulated water molecules with different arrangements. The result was that without the pyramid structure, water would lose all its special properties and become a normal liquid.
Mr. John Russo, head of the research project, said:"Through this experiment, we know thatThe tetrahedral molecular structure is the reason why water has properties different from other natural liquids.".
"We hope that the results of this work will contribute to explaining the mysteries of water."– he added.
The study was published by the National Academy of Sciences (USA).
In addition, water has many interesting things that we do not pay attention to. Let's read the article Interesting things about water that you may not know to discover these interesting things.



