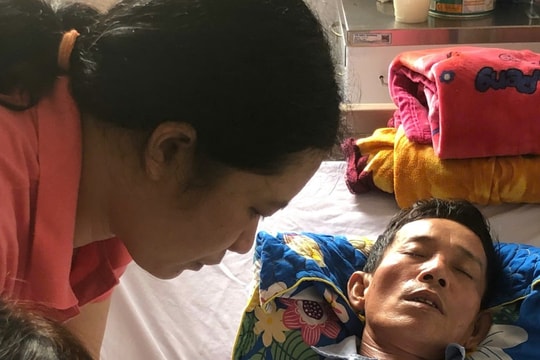
US allows gene editing to treat cancer for the first time
The US government has just approved a breakthrough method to treat blood cancer by adjusting the body's immune system to fight cancer.
This method is considered more effective than mass drug use because it is based on each person's own defense mechanism.
The US Food and Drug Administration (FDA) announced that it had made a “historic” decision to approve this bold gene therapy to advance “a new approach to treating cancer and other serious, life-threatening diseases.”
The method, called Kymiah, by pharmaceutical company Novartis, is advertised as a one-time, definitive treatment delivered through a vein.
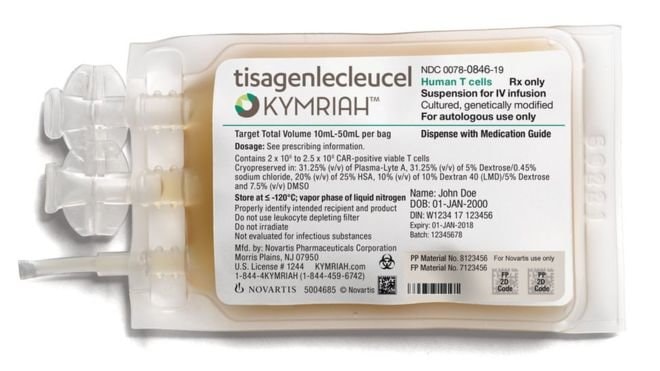 |
| The new drug will be "tailored" for each patient - Photo: Novartis |
Accordingly, Novartis' people will "redesign" the genes of immune cells taken from the patients' own blood and turn them into "killers" that hunt down cancer cells.
The “living drug” called CAR-T targets acute lymphoblastic leukemia — the most common childhood cancer in the United States.
Initial results were quite promising, with 83% of people treated with this method being completely cured of cancer within the first three months.
Current treatments, such as surgery and chemotherapy, help 85 percent of children with cancer stay in remission for five years or longer, according to the American Cancer Society.
It is unclear what the long-term consequences of gene editing will be.
But for now, the pharmaceutical company Novartis is charging up to $475,000 for this treatment, although it says it will not charge the patient if there is no response within the first month.
The above-mentioned high price has made many people worry that it could create a generation of super-expensive treatment drugs that not everyone can afford.
David Mitchel, president of Patients for Affordable Medicines, criticized Novartis for bringing expensive drugs to market “so they can charge more money to people.”
This method can also only treat a few hundred patients a year. In addition, it has difficulty treating “hard tumors” such as lung cancer and melanoma. Some side effects such as cytokine release are dangerous, although they can be controlled with drugs.
However, the new method has been praised by the scientific community. "We have never seen anything like this before," said Dr. Stephan Grupp of the Children's Hospital of Philadelphia, which is collaborating with Novartis, as he said a girl who nearly died of cancer was now in remission thanks to the new method.
 |
| New treatments are still expensive. Pictured inside a cancer research lab in the UK. Photo: Reuters |
“We believe this is just the beginning of many immunotherapy-based treatments for many different cancers that will soon be available,” said Dr. David Maloney of the Fred Hutchinson Cancer Research Center.
“We are entering a new frontier in revolutionary medicine with the ability to manipulate a patient's own blood cells to attack a deadly cancer,” the FDA said in its review of the first gene-editing therapy it has approved.
According to TTO
| RELATED NEWS |
|---|




.jpg)