Recall of Dodextro capsules for pain relief and fever reduction
Dodextro capsules with pain-relieving, fever-reducing, and anti-inflammatory effects have been suspended from circulation and recalled nationwide due to substandard test results.
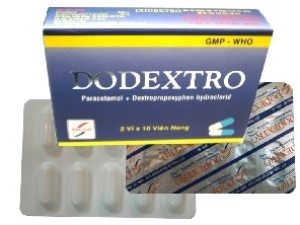 |
The Drug Administration of Vietnam (Ministry of Health) said that the Dodextro capsule drug that was suspended from circulation has production batch: 213904, expiry date: February 7, 2015, registration number: VD-3004-07, produced by Dong Nam Pharmaceutical Production and Trading Joint Stock Company.
Previously, the Ho Chi Minh City Drug Testing Institute took drug samples at booth 4, Codupha Pharmaceutical Center, 334 To Hien Thanh, Ward 14, District 10, Ho Chi Minh City for testing. The results showed that the drug did not meet the quality standards for the qualitative index of Dextropropoxyphene. Therefore, the entire batch of the drug was forced to suspend circulation and recall nationwide.
The Drug Administration of Vietnam requests the manufacturing company to promptly send a recall notice to the distributors and users of Dodextro capsules produced by the company and recall all batches of the drug that do not meet the above quality standards.
The Department of Health of provinces and centrally run cities, and health sectors shall notify drug trading and using establishments to recall the above batch of substandard drugs, inspect and supervise the units carrying out the above recall, and handle violators according to current regulations.
According to HNMO - TH






