Parents need to know: Information about 2 types of COVID-19 vaccines that will be injected into children from 5 to under 12 years old
(Baonghean.vn) - There are 2 types of vaccines that have been decided to be injected into children of this age. Vaccination and pediatric experts recommend not to mix the 2 types...
According to the Ministry of Health, by early April 2022, COVID-19 vaccination will be implemented for children aged 5 to under 12 years old.
Associate Professor, Dr. Duong Thi Hong - Deputy Director of the National Institute of Hygiene and Epidemiology stated that from the beginning of April 2022, immediately after the COVID-19 vaccine is supplied, all 63 provinces and cities across the country will deploy vaccination.COVID-19 vaccinefor children from 5 to under 12 years old.
Accordingly, there are 2 types of vaccines: Moderna Vaccine and Pfizer Vaccine will be injected for children from 5 to under 12 years old. However, the injection dose of each injection of the 2 different vaccines, the age of injection is also different. Here is information about these 2 types of vaccines.
Pfizer vaccine is used for children from 5 to under 12 years old.
This vaccine was conditionally approved by the Ministry of Health for urgent needs in COVID-19 prevention and control in Decision No. 457/QD-BYT dated March 1, 2022.
The Pfizer vaccine used for children aged 5 to under 12 years has a dose of 10mcg, which is 1/3 the dose used for people aged 12 years and older. The injection dose is 0.2ml (each 0.2ml dose contains 10mcg of mRNA COVID-19 vaccine). This vaccine is injected intramuscularly. The injection schedule is 2 doses, 3 weeks apart.
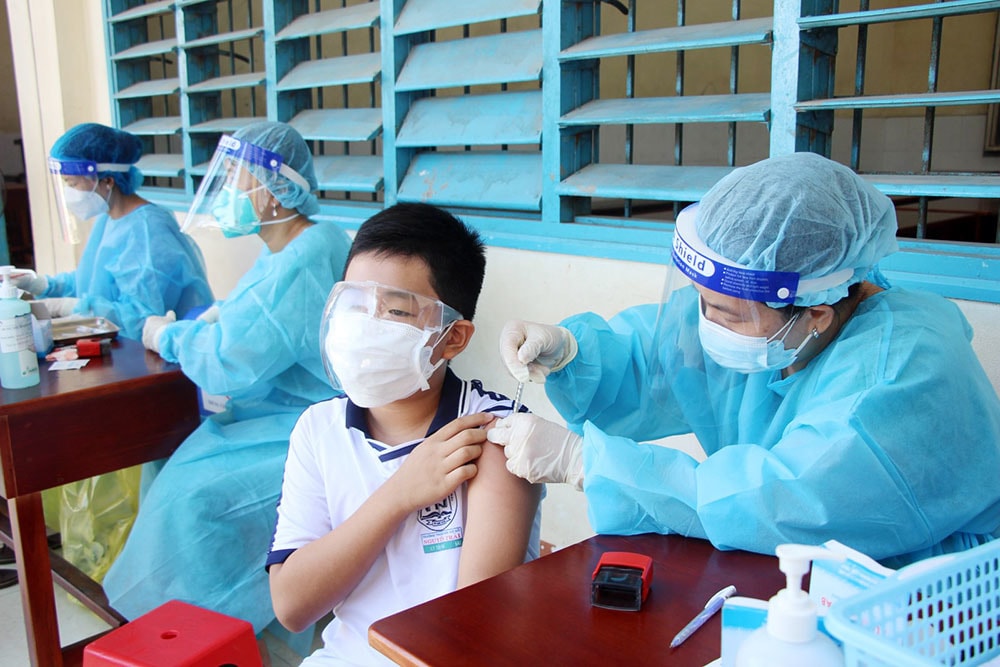 |
| The Prime Minister has repeatedly directed that all conditions be prepared for vaccination of children. Photo courtesy |
Packaging: 1 tray contains 195 vials; each vial contains 10 doses, or 1 box contains 10 vials; each vial contains 10 doses.
Store at deep freezing temperatures from -90ºC to -60ºC, shelf life 9 months from date of manufacture
Store at +2ºC to +8ºC maximum shelf life 10 weeks.
Thawed vaccines should not be returned to sub-zero temperatures.
For the Pfizer vaccine, very common reactions when vaccinating children aged 5- under 12 years against COVID-19 are: Headache, diarrhea, joint pain, muscle pain, pain at the injection site, exhaustion, chills, fever (higher frequency for the second dose), swelling at the injection site (> 80%), exhaustion (> 50%), headache (> 30%), redness and swelling at the injection site (> 20%), muscle pain and chills (> 10%).
Unlike people aged 18 and over, for children aged 5 - under 12, the Ministry of Health requires only 2 single injections of the same vaccine, not mixed with any mRNA vaccine.
"This reaction also occurs in children aged 12-17 years old when vaccinated against COVID-19," said Ms. Hong.
The most common reactions in the age group 5 - under 12 years are: nausea, redness at the injection site.
Rare reactions include lymphadenopathy, hypersensitivity reactions (rash, itching, urticaria, angioedema), decreased appetite, insomnia, lethargy, increased sweating, night sweats, pain in the extremities, asthenia, malaise, and itching at the injection site.
Moderna vaccine for children from 6 to under 12 years old
On March 31, the Ministry of Health approved the Moderna vaccine for children aged 6 to under 2 years old. Intramuscular injection, the dose is half the basic adult dose (equivalent to 0.25 ml), similar to the booster dose for adults. 2 injections are given 4 weeks apart.
Moderna vaccine in multi-dose vials: 10 doses per 0.5ml vial equivalent to 20 doses per 0.25ml dose.
Store at -25⁰C to -15⁰C, shelf life 9 months from date of manufacture.
Store at temperature +2⁰C to +8⁰C, use within 30 days.
Very rare reactions are myocarditis and pericarditis (less than 1 in 10,000). However, this reaction has not been recorded in children aged 12-17 years who have been vaccinated against COVID-19 in the system.
For the Moderna vaccine: very common reactions are: Swollen lymph nodes on the same side as the injection site, some cases of swollen lymph nodes in other areas (e.g., in the neck, above the collarbone), headache, nausea/vomiting, muscle pain, joint pain, pain at the injection site, fatigue, chills, fever, swelling at the injection site, erythema at the injection site.
The most commonly reported adverse reactions in children aged 6–12 years after the primary vaccination course were injection site pain (98.4%), fatigue (73.1%), headache (62.1%), myalgia (35.3%), chills (34.6%), nausea/vomiting (29.3%), armpit swelling/pain (27.0%), fever (25.7%), injection site erythema (24.0%), injection site swelling, and arthralgia;
Common reactions are: Diarrhea, rash, urticaria at injection site, rash at injection site, delayed reaction at injection site.
Rare reactions are: Dizziness, itching at the injection site.
Rare reactions are: Decreased sensation, facial swelling in people with a history of dermal filler injections;
Very rare reactions are: Myocarditis, pericarditis.
As of March 15, according to reports from the World Health Organization (WHO) and the manufacturer, 66 countries around the world have licensed the circulation and use of the Pfizer vaccine for children aged 5-11.
Some countries that have implemented typical vaccinations are the US, Australia, Singapore, Japan, South Korea, and Malaysia.
Many European countries have also vaccinated children in this age group with both Pfizer and Moderna vaccines.