Ministry of Health adds drugs to Covid-19 treatment regimen
According to the latest guidelines, the Ministry of Health has added oral and injectable antiviral drugs and some monoclonal antibody drugs for mild Covid-19 patients and people with underlying diseases.
The Ministry of Health has just issued a decision on amending and supplementing the Guidelines for Diagnosis and Treatment.Covid-19 treatmentcaused by the new strain of Corona virus (SARS-CoV-2). In this new guideline, the Ministry of Health adds antiviral drugs in the treatment of Covid-19 to inhibit virus replication.
Accordingly, the Ministry of Health stipulates that antiviral drugs are drugs that inhibit viral replication. Oral antiviral drugs are often used for all cases of early-stage SARS-CoV-2 infection. Injectable and infusion antiviral drugs are often used for inpatients.
The decision clearly states that for drugs that have not been recommended for use by the World Health Organization, have not been licensed for circulation, or have not been granted emergency use authorization in any country in the world, their use must comply with the clinical trial regulations of the Ministry of Health.
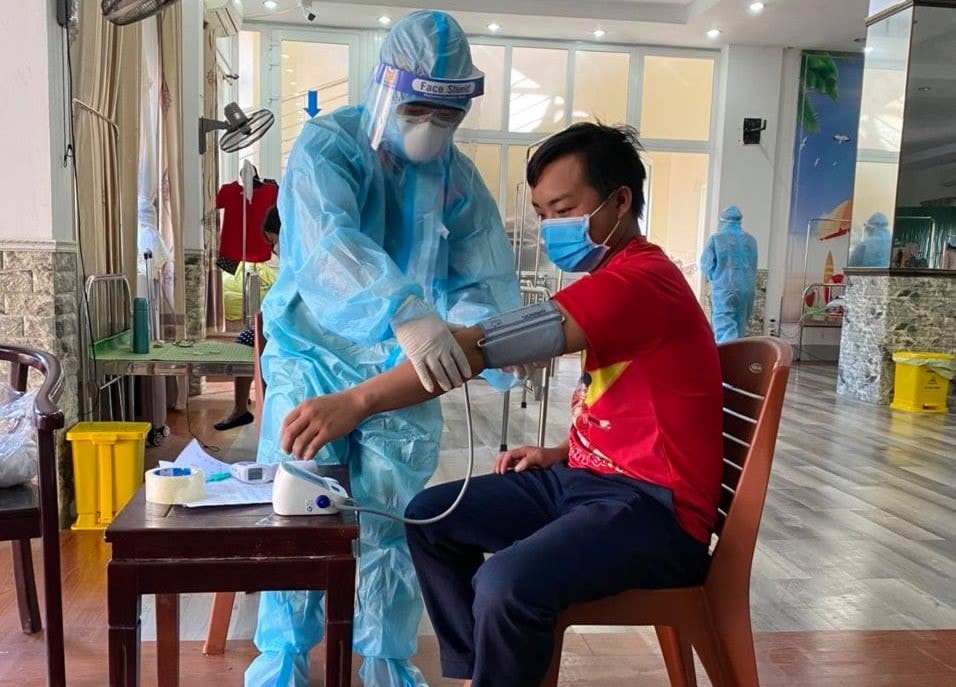 |
| Doctors examine an F0 in Field Hospital No. 4. Photo TH |
Drugs that have been recommended for use by the World Health Organization or licensed for circulation, or licensed for emergency use in at least one country in the world are allowed to be used according to the manufacturer's instructions for use included when imported.
Also in this latest guideline, the Ministry of Health also added monoclonal antibody drugs (which are Interleukine 6 inhibitors or virus neutralizers).
This drug is indicated for the treatment of patients aged 12 years and older with confirmed mild to moderate SARS-CoV-2 infection and at risk of severe progression such as the elderly, obese, cardiovascular disease, hypertension, chronic lung disease, type 1 and type 2 diabetes, chronic kidney disease, including patients on dialysis, chronic liver disease, immunodeficiency undergoing cancer treatment, bone marrow or organ transplant, immunodeficiency, HIV, sickle cell anemia, thalassemia and long-term use of immunosuppressive drugs.
For drugs that have not been recommended for use by the World Health Organization, have not been licensed for circulation, or have not been granted emergency use authorization in any country in the world, their use must comply with the clinical trial regulations of the Ministry of Health.






.jpg)
