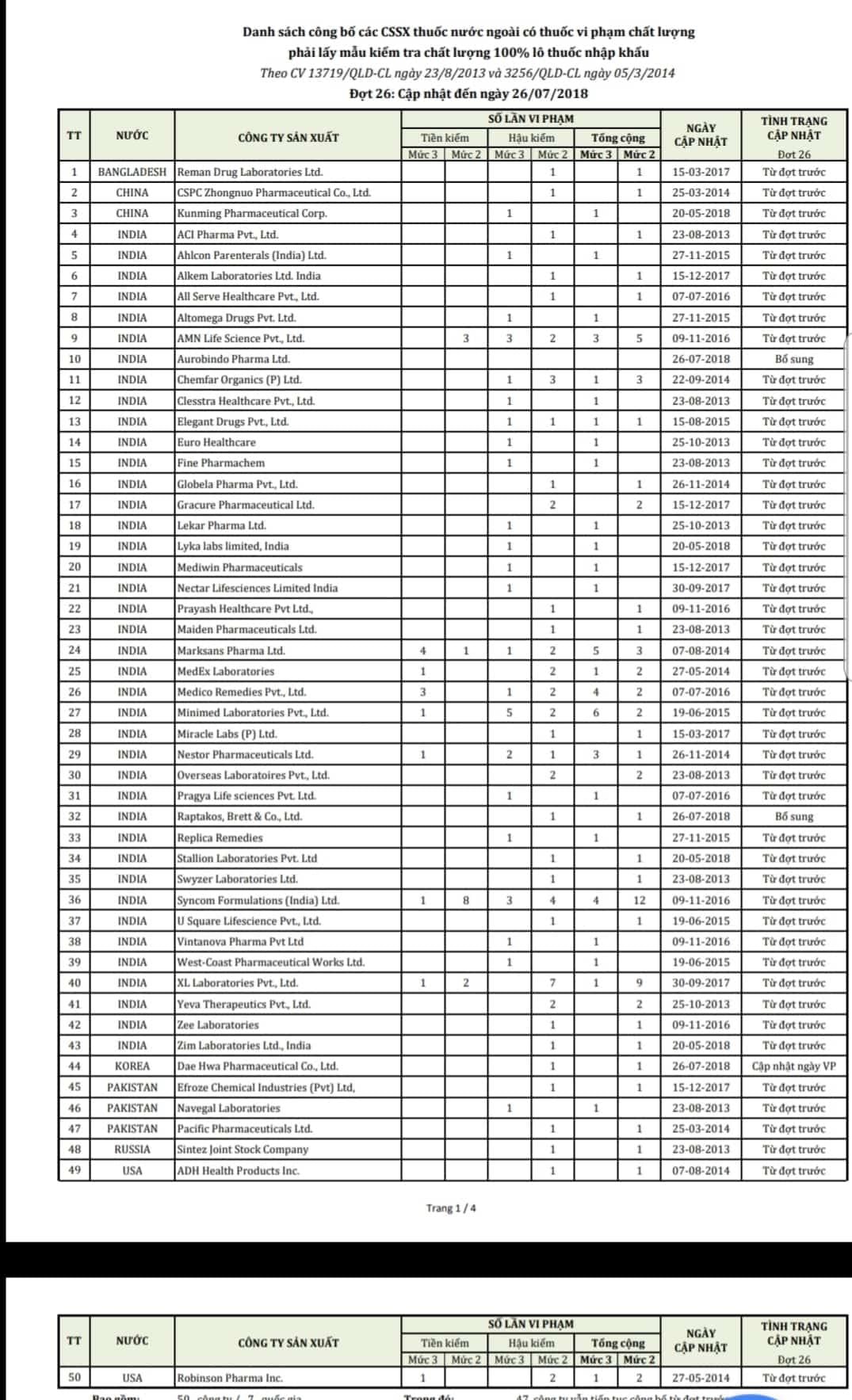
Ministry of Health announces 50 foreign companies with drugs violating quality
The Drug Administration of Vietnam, Ministry of Health has just announced a list of 50 foreign companies with drugs that violate quality standards, and must conduct quality testing on 100% of imported drug batches.
|
| List of 50 foreign companies with drugs violating quality. |
Based on the results of quality testing of imported drugs according to regulations, the Drug Administration of Vietnam, Ministry of Health has just sent an official dispatch to the Department of Health of provinces and cities and drug import-export companies announcing a list of 50 foreign companies with drugs violating quality standards, requiring them to take samples for quality testing of 100% of imported drug batches.
These pharmaceutical companies belong to 7 countries including India (39 enterprises), Pakistan, China, South Korea, Russia, the US and Bangladesh.
In this announcement, most of the companies had drug quality violations from previous inspections, so they had to take samples for quality testing on 100% of imported drug batches instead of post-inspection as prescribed.
The Drug Administration of Vietnam requests the Department of Health of provinces and centrally-run cities to direct drug inspection, management and testing units to conduct inspections and supervision of compliance with regulations on quality control of imported drugs in circulation in the management area and handle organizations and individuals who violate the regulations.
According to the Drug Administration of Vietnam, before being put on the market, the Ministry of Health must review the registration dossier, including raw materials, production processes, quality standards, stability and clinical data. The manufacturer must meet the manufacturing conditions (GMP), must strictly comply with the approved drug registration dossier during the production process and must check the quality to meet the registered standards before putting the drug on the market.
When putting drugs into circulation on the market, the manufacturing/importing facility self-monitors and is responsible for the quality of the drugs it produces, reports to the management agency when detecting signs of potential risks to the health of users, and is subject to sampling and supervision by the management agency.
Every year, testing systems take about 40,000 drug samples on the market to monitor quality (in 2015, 38,627 samples were taken, in 2016, 37,219 were taken, in 2017, 36,362 were taken). The rate of poor quality drugs in recent years is about 1.5 - 2.0% and the rate of fake drugs is less than 0.05%./.






.jpg)
