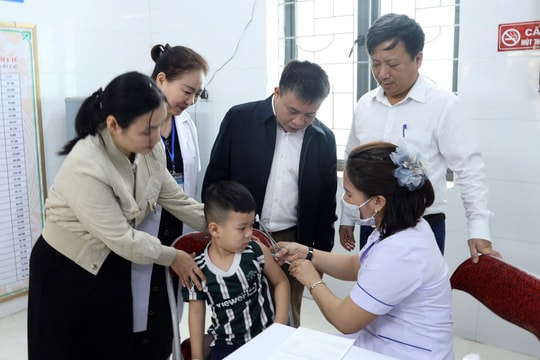
The first 117,000 doses of Covid-19 vaccine arrived in Vietnam
According to information from the Ministry of Health, this morning, the first 117,000 doses of Covid-19 vaccine arrived at Tan Son Nhat airport.
Today, the flight carrying the first doses of AstraZeneca's Covid-19 vaccine landed at Tan Son Nhat Airport in Ho Chi Minh City, bringing new hope to Vietnam's fight against the pandemic.
The shipment includes 117,600 doses of vaccine.Covid-19 vaccineThe first batch of the vaccine in the country was welcomed by Dr. Truong Quoc Cuong - Deputy Minister of Health, along with representatives of the Departments, Bureaus, and Institutes under the Ministry of Health; representatives of AstraZeneca Vietnam Co., Ltd. and Vietnam Vaccine Joint Stock Company (VNVC); and Ms. Emily Hamblin, British Consul General in Ho Chi Minh City.
AstraZeneca's Covid-19 vaccine was approved for urgent needs in preventing and fighting the Covid-19 epidemic in Vietnam in early February 2021.
 |
Prof. Dr. Nguyen Thanh Long, member of the Party Central Committee and Minister of Health, said: “The first doses of AstraZeneca Covid-19 vaccine have arrived in time while the whole country is responding to the new outbreak. We will prioritize vaccination for frontline forces and subjects according to Government regulations; currently, the Ministry of Health is making efforts to negotiate with other vaccine manufacturers to provide enough vaccines for people in 2021 according to the direction of the Politburo.
This vaccine will help us move one step closer to overcoming the Covid-19 pandemic and bringing life back to normal. We appreciate the active cooperation of all relevant agencies, ministries and sectors, AstraZeneca Vietnam and VNVC, which has helped Vietnam become one of the first countries in Asia to access the Covid-19 vaccine."
Mr. Nitin Kapoor, Chairman and CEO of AstraZeneca Vietnam, said: “We are grateful for the proactive vaccine access strategy and the trust of the Vietnamese Government and the Ministry of Health in making it possible for the vaccine batch to be available in Vietnam soon. As per the guidelines, these first doses will undergo the final quality control process before being handed over to the Ministry of Health/VNVC to start vaccination for priority groups.
After 27 years of association with Vietnam, AstraZeneca is proud to make a meaningful contribution to the fight against Covid-19 and the national economic recovery through the supply of this vaccine. We will continue to work closely with the Government, the Ministry of Health and VNVC to provide vaccines to people as quickly and safely as possible."
 |
British Ambassador to Vietnam Gareth Ward said: “The AstraZeneca and Oxford University Covid-19 vaccine is a great example of UK science and innovation. I am delighted that it is now available in Vietnam for the national vaccination programme. Science and collaboration will help us beat the pandemic.”
The vaccine was shown to be well tolerated and effective in preventing symptomatic Covid-19. After the first dose, the vaccine provided 76% protection for 90 days, with no significant decline in protection over this period. Vaccine efficacy after the second booster dose was higher with a longer interval than the first dose, reaching 81% when the interval between the two doses was 12 weeks or more.
Results from a clinical trial showed that the AstraZeneca Covid-19 vaccine provided 100% protection against severe disease, hospitalization and death from Covid-19 from 22 days after the first dose. The analysis also showed that the vaccine had the potential to reduce the risk of asymptomatic transmission of the virus by two-thirds. This significant proportion was determined based on weekly nasal swab samples collected from volunteers in the UK trial.
The AstraZeneca Covid-19 vaccine can be stored, transported and handled at normal refrigerated conditions (2-8oC) for at least six months, allowing for easy use within existing healthcare facilities.
This batch of vaccine is produced by AstraZeneca through a global supply chain. To ensure broad and equitable access to the vaccine, in 2020, AstraZeneca built more than ten regional supply chains, leveraging its own capabilities and collaborating with more than 20 partners to accelerate vaccine production and supply.
AstraZeneca's Covid-19 vaccine has been granted conditional marketing authorization or emergency use approval in more than 50 countries. The World Health Organization (WHO) has also granted emergency use authorization for the vaccine to speed up access to vaccines for more than 145 countries through the COVAX Facility, the vaccine procurement and supply activity under the "Global Access to Covid-19 Vaccines (COVAX)" mechanism.