Vietnam uses Indian vaccine to replace Quinvaxem
From next June, Vietnam will use India's ComBE Five vaccine to replace the 5-in-1 Quinvaxem vaccine.
This is the information given by Associate Professor, Dr. Tran Nhu Duong, Deputy Director of the National Institute of Hygiene and Epidemiology at a media conference on some new vaccines in the 2018 expanded immunization program.
Associate Professor Duong said that the Quinvaxem combination vaccine of Berna Biotech (Korea) has been used in the expanded immunization program since 2010 with about 41 million doses.
However, this vaccine has stopped production, the remaining Quinvaxem vaccines will be injected until the end of May.
|
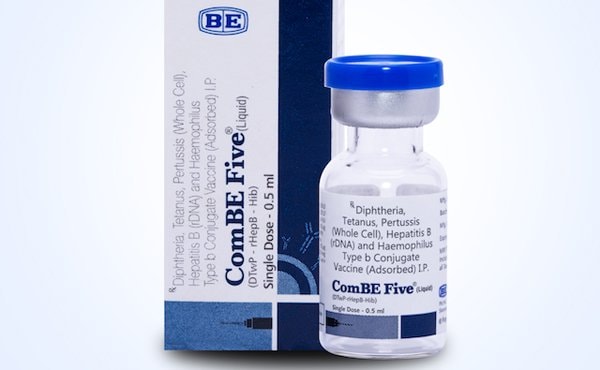
| ComBE Five vaccine will be included in the expanded vaccination program from next June. |
From next June, Vietnam will introduce India's ComBE Five vaccine into its expanded immunization program. This vaccine has been licensed for circulation in Vietnam since May 30, 2017 and is valid for 5 years.
According to Associate Professor Duong, ComBE Five vaccine has the same ingredients as Quinvaxem, preventing five diseases: diphtheria, whooping cough, tetanus, hepatitis B and meningitis caused by Hib bacteria.
This vaccine has been circulating in India since 2010, has been prequalified by WHO since 2012 and has administered more than 400 million doses in 43 countries. India alone has administered 130 million doses under its expanded immunization program.
“Before being injected nationwide, the ComBE Five vaccine was supervised by the Military Medical Academy for a pilot injection in four districts of Ha Nam from September 2016 to January 2017 and was accepted by the Ministry of Health’s scientific council. It was then injected on a pilot basis in Binh Dinh, Kon Tum and Dong Thap,” Associate Professor Duong informed.
After injection, only some common reactions were recorded on the first day after vaccination, including injection site reactions with pain and redness at a rate of 5-15%, 34-39% fever, no serious reactions were recorded after vaccination.
Statistics on over 40 million doses of ComBE Five vaccine in India show only 11 cases of severe reactions after vaccination, including 6 deaths.
When the cause was carefully examined, none of the cases were related to the vaccine (1 death due to pneumonia, 1 unknown cause, 1 case of sudden infant death syndrome, 1 death due to sepsis, 2 deaths due to milk aspiration).
In addition, each batch of vaccines imported into Vietnam is inspected by the National Institute for Control of Vaccines and Biologicals and meets safety standards before being put into use.
Regarding the vaccination schedule, children who have received the first and second doses of Quinvaxem will receive ComBE Five in the next dose without needing to be re-vaccinated.