WHO gives 'green light' to AstraZeneca
(Baonghean.vn) - Earlier this week, the World Health Organization (WHO) granted emergency use authorization for AstraZeneca's new coronavirus (Covid-19) vaccine. This move is expected to pave the way for partners of this United Nations organization to deliver millions of doses to countries under an initiative to equitably distribute vaccines and quickly control the pandemic.
Open up hope
According to the AP news agency, in a statement released on February 15,WHOThe agency said it had approved vaccines made by the Serum Institute of India and South Korea's AstraZeneca-SKBio. This is the second time the UN health agency has approved other Covid-19 vaccines, following the Pfizer-BioNTech vaccine last December. The green light for the AstraZeneca vaccine also means the distribution of hundreds of millions of doses to countries that have signed up to participate in the UN COVAX initiative, which aims to get vaccines to the most vulnerable people around the world.
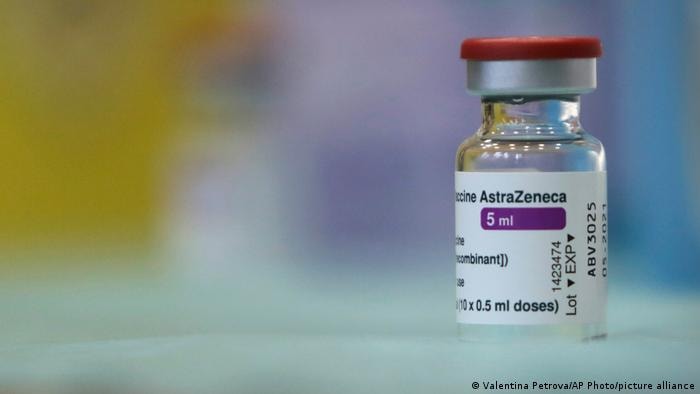 |
| AstraZeneca is the first vaccine to receive emergency approval from the WHO for global distribution through the COVAX initiative. Photo: AP |
“Countries that have so far lacked access to vaccines will finally be able to start vaccinating health workers and high-risk populations,” said Dr. Mariângela Simão, WHO Assistant Director-General for Access to Medicines and Health Products.
Since its first appearance, Covid-19 has “stormed” everywhere, infecting more than 109 million people worldwide and killing at least 2.4 million of them. But, many countries are still unable to start vaccination programs, and even rich countries are now facing shortages.VaccineAlthough companies are racing to increase production capacity.
Back to AstraZeneca - this company's vaccine has now been licensed in more than 50 countries worldwide, including countries such as the UK, India, Argentina and Mexico. Compared to the Pfizer-BioNTech vaccine, the AstraZeneca vaccine has the "advantage" of being more reasonably priced. Not to mention that the preservation of the AstraZeneca vaccine is also simpler, does not require storage in deep cold conditions like the Pfizer-BioNTech vaccine, "making it difficult" for preservation technology in many developing countries. However, the "common point" of these two vaccines is that each subject needs to be fully vaccinated with 2 doses, several weeks apart, to ensure effectiveness.
Last week, WHO vaccine experts recommended the AstraZeneca vaccine for people aged 18 and over, even in countries where variants of Covid-19 have been detected. However, the Africa Centers for Disease Control and Prevention has advised that countries where the virus variant first identified in South Africa should exercise “caution” in using the AstraZeneca vaccine and instead prioritize other vaccines.
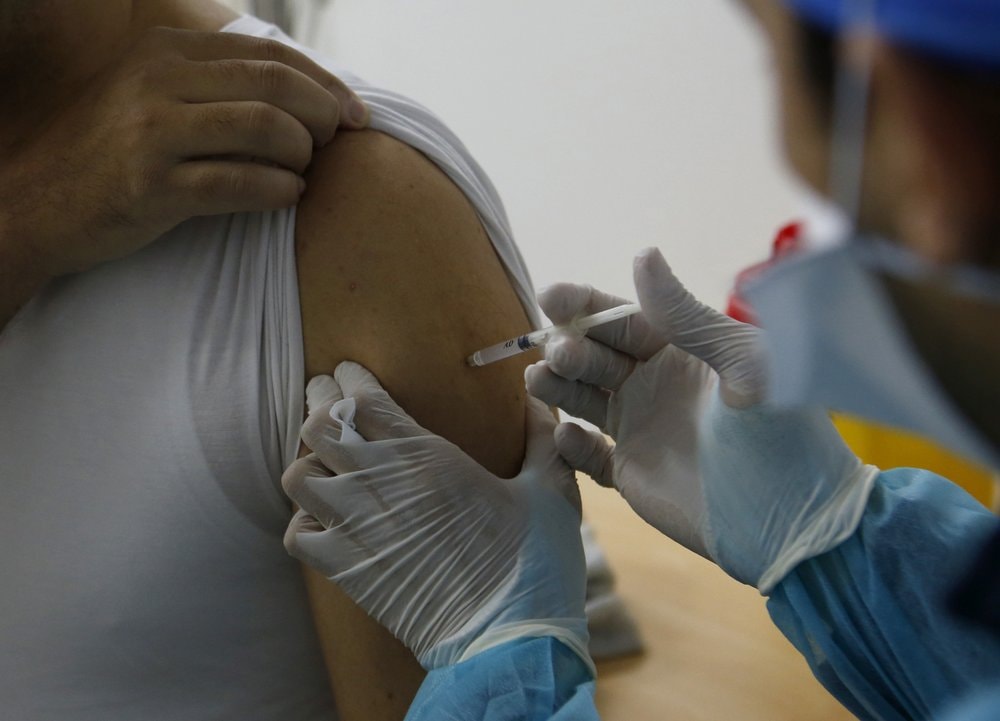 |
| AstraZeneca Covid-19 vaccine injection for medical staff in Morocco. Photo: AP |
In fact, the AstraZeneca vaccine makes up the bulk of COVAX’s stock, and concerns have recently arisen after preliminary research suggested it may not prevent mild to moderate disease caused by the South African variant. Last week, South Africa scaled back its AstraZeneca vaccination program, opting instead for a different, unlicensed vaccine from Johnson & Johnson to vaccinate its health care workers.
The goal of equitable distribution
COVAX - a United Nations initiative that can be said to have "missed" its stated goal of launching programsCorona virus vaccinationin poor countries at the same time as rich ones. In recent weeks, many developing countries have rushed to sign private deals to buy vaccines, rather than wait for allocations from COVAX.
WHO and its partners, including the GAVI vaccine alliance, have not yet revealed which countries will receive the first doses from the COVAX program. But a preliminary plan shows that many rich countries that have signed separate vaccine agreements, such as Canada, South Korea, New Zealand, etc., are also on the list to receive vaccines from COVAX early.
Some public health experts say this is “very problematic” and explain that this is a flaw in the COVAX calculation, as the initiative allows donor countries to buy vaccines from the program while also signing their own commercial contracts. For example, Anna Marriott, head of health policy for Oxfam International, said: “Canada ordered more than five times the number of doses that would have been enough to cover their own population and now they are waiting for their share from COVAX that should have gone to poor countries.”
 |
| British Prime Minister Boris Johnson holds a vial of the AstraZeneca vaccine during a visit to the Covid-19 Vaccine Production Centre in Orpington, Southeast London. Photo: AP |
Indeed, after pledging more than $400 million to COVAX last year, Prime Minister Justin Trudeau affirmed that the maple leaf country always intended to receive more vaccines through the COVAX mechanism. On this issue, WHO chief scientist Dr. Soumya Swaminathan said that if rich countries signed up to receive vaccines from COVAX, those requests would not be rejected. "The COVAX mechanism will not punish countries," she said in early February.
However, experts like Marriott still believe that rich countries that are planning to receive vaccine doses from COVAX should reconsider, as previous calls were made to support the initiative's goal of ensuring equitable access to vaccines for all countries in the world, regardless of rich or poor, rich or poor... "Rich countries that already have their own vaccine supply should not deprive countries in dire straits of doses that should be reserved for countries in dire need," Marriott argued.